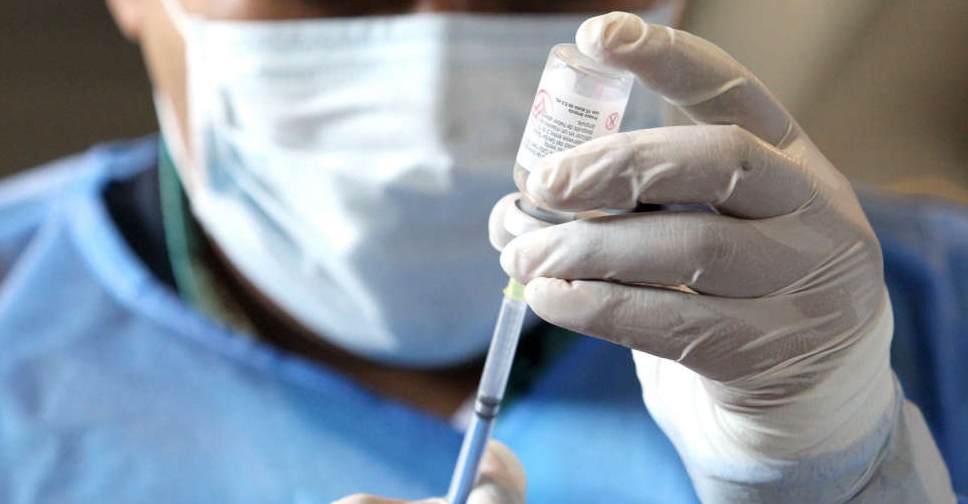
The World Health Organization (WHO) on Thursday issued an emergency use listing for the single-dose COVID-19 vaccine from China-based CanSino Biologics.
The vaccine, Convidecia, is the eleventh shot against the coronavirus to get clearance from the global health agency, whose advisory group recommended its use in people of age 18 years and above.
It was found to have 64 per cent efficacy against symptomatic disease and 92 per cent against severe COVID-19, the agency said.
Other vaccines that have similar clearance from the agency include those made by Pfizer and BioNTech, Moderna, AstraZeneca, Johnson & Johnson and Novavax.




 Azerbaijan vows to respond after four injured by Iranian drones
Azerbaijan vows to respond after four injured by Iranian drones
 72 killed in Israeli attacks on Lebanon as it warns residents to leave south
72 killed in Israeli attacks on Lebanon as it warns residents to leave south
 Nepal goes to the polls; voters seek change after youth-led protests
Nepal goes to the polls; voters seek change after youth-led protests
 Landslide kills over 200 people at Congo's Rubaya mine
Landslide kills over 200 people at Congo's Rubaya mine
 80 people killed after US sinks Iranian warship
80 people killed after US sinks Iranian warship



