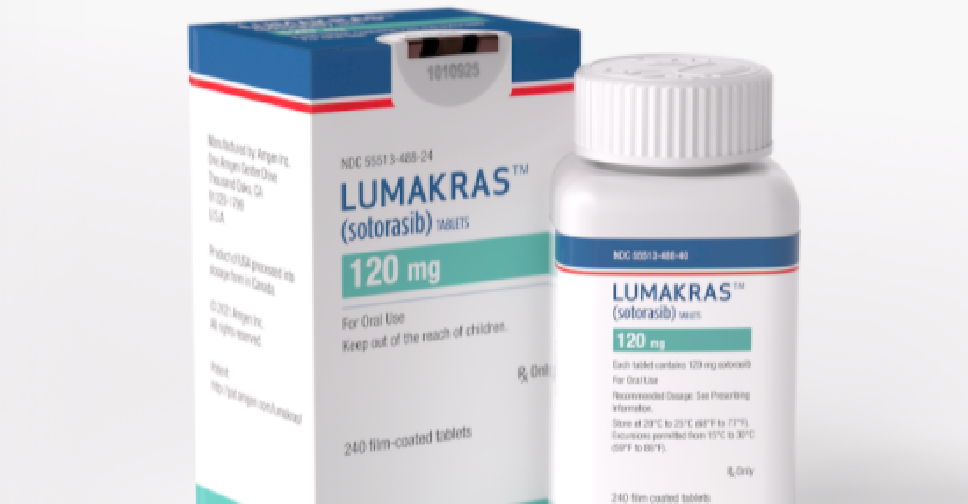
The UAE has become the second country in the world to approve the new lung cancer drug 'Lumakras'.
The Ministry of Health and Prevention (MoHAP) has cleared the registration and use of the drug, which recently received the approval of the US Food and Drug Administration (FDA).
It will help speed up the treatment plan of patients in the UAE and improve their quality of life.
Lumakras, manufactured by Amgen, is prescribed to adult patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC), who have received at least one previous cancer therapy.
It is supplied as film-coated tablets for oral use containing 120 mg of sotorasib.



 New UAE, Bahrain fast-track travel system takes off
New UAE, Bahrain fast-track travel system takes off
 Dubai adjusts paid parking hours for Ramadan
Dubai adjusts paid parking hours for Ramadan
 Dubai's Al Jalila Foundation unveils cancer support fund
Dubai's Al Jalila Foundation unveils cancer support fund
 Dubai Police urge vigilance against online begging
Dubai Police urge vigilance against online begging
 H.H. Sheikh Hamdan pays tribute to UAE's royal photographer
H.H. Sheikh Hamdan pays tribute to UAE's royal photographer
 Sharjah approves over AED76 million in debt settlement for citizens
Sharjah approves over AED76 million in debt settlement for citizens
 H.H. Sheikh Hamdan awards Arab Hope Makers
H.H. Sheikh Hamdan awards Arab Hope Makers
 UAE looks into framework to regulate children's social media use
UAE looks into framework to regulate children's social media use
 UAE Ramadan moon-sighting committee to meet on Tuesday
UAE Ramadan moon-sighting committee to meet on Tuesday
 RTA opens second bridge at Al Qudra intersection
RTA opens second bridge at Al Qudra intersection
 Abu Dhabi identifies over 40 modern heritage sites
Abu Dhabi identifies over 40 modern heritage sites
 Distribution of aid from UAE's Saqr Humanitarian Ship begins in Gaza
Distribution of aid from UAE's Saqr Humanitarian Ship begins in Gaza
 Dubai to expand bus and taxi lanes as public transport ridership grows by 7.4%
Dubai to expand bus and taxi lanes as public transport ridership grows by 7.4%
 Emir of Qatar welcomed by UAE President on visit to Abu Dhabi
Emir of Qatar welcomed by UAE President on visit to Abu Dhabi
 UAE, Egypt Foreign Ministers discuss Gaza peace plan
UAE, Egypt Foreign Ministers discuss Gaza peace plan
 UAE, Pakistan leaders discuss boosting economic ties
UAE, Pakistan leaders discuss boosting economic ties
 UAE, Bosnia FMs discuss strengthening bilateral relations
UAE, Bosnia FMs discuss strengthening bilateral relations
 H.H. Sheikh Mohammed launches campaign to rescue 5 million children from hunger
H.H. Sheikh Mohammed launches campaign to rescue 5 million children from hunger
 Dubai advances measures to support people of determination
Dubai advances measures to support people of determination
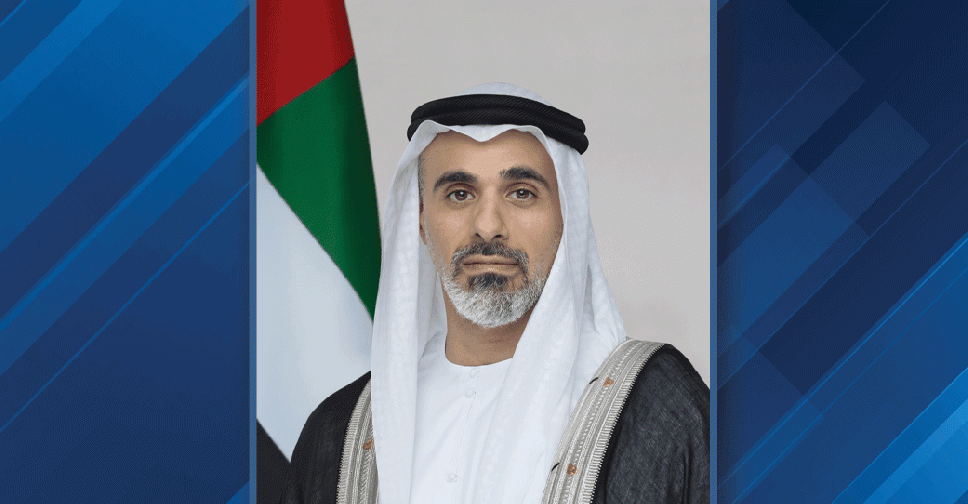 Abu Dhabi Crown Prince to lead UAE delegation at AI Impact Summit in India
Abu Dhabi Crown Prince to lead UAE delegation at AI Impact Summit in India
