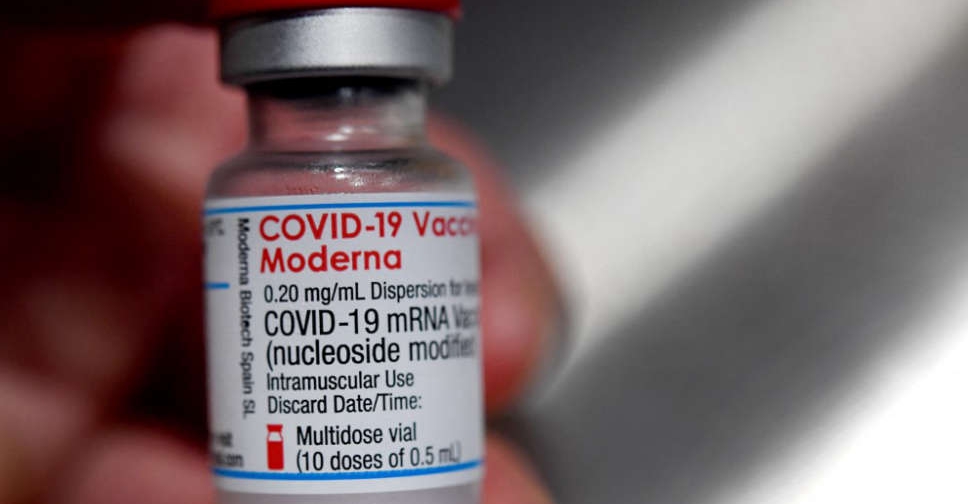
The UAE's Ministry of Health and Prevention has announced the registration of Moderna's COVID-19 vaccine for emergency use.
It's the fifth COVID-19 vaccine to be approved for use in the country.
The decision was made following the completion of clinical trials and a strict assessment, as well as the approval of the US Food and Drug Administration (FDA).
Dr. Mohamed Salim Al-Olama, Undersecretary of the Mohap, highlighted that the move is crucial to accelerate the pace of recovery and community immunization.
The decision enables health authorities to import the vaccine after fulfilling shipping-related safety and efficacy standards, Dr Amin Hussein Al Amiri added.
Currently, four vaccines are available in the UAE - Pfizer-BioNTech, Oxford AstraZeneca, Sputnik V and Sinopharm.



 UAE confirms first day of Ramadan
UAE confirms first day of Ramadan
 Dubai expands road network with new Al Rowaiyah corridor
Dubai expands road network with new Al Rowaiyah corridor
 UAE extends Emirates Mars Mission until 2028
UAE extends Emirates Mars Mission until 2028
 UAE joins 7 nations in condemning Israel's West Bank land registration plan
UAE joins 7 nations in condemning Israel's West Bank land registration plan
 Dubai Holding, Nord Anglia Education partner to develop new premium schools
Dubai Holding, Nord Anglia Education partner to develop new premium schools
 UAE President posts special message on Lunar New Year
UAE President posts special message on Lunar New Year
 New UAE, Bahrain fast-track travel system takes off
New UAE, Bahrain fast-track travel system takes off
 Dubai adjusts paid parking, Salik hours for Ramadan
Dubai adjusts paid parking, Salik hours for Ramadan
 Dubai's Al Jalila Foundation unveils cancer support fund
Dubai's Al Jalila Foundation unveils cancer support fund
 Dubai Police crowned champions of UAE Rescue Challenge
Dubai Police crowned champions of UAE Rescue Challenge
 UAE discusses strengthening partnership with NATO
UAE discusses strengthening partnership with NATO
 Dubai Police urge vigilance against online begging
Dubai Police urge vigilance against online begging
 H.H. Sheikh Hamdan pays tribute to UAE's royal photographer
H.H. Sheikh Hamdan pays tribute to UAE's royal photographer
 Sharjah approves over AED76 million in debt settlement for citizens
Sharjah approves over AED76 million in debt settlement for citizens
 H.H. Sheikh Hamdan awards Arab Hope Makers
H.H. Sheikh Hamdan awards Arab Hope Makers
 UAE looks into framework to regulate children's social media use
UAE looks into framework to regulate children's social media use
 UAE Ramadan moon-sighting committee to meet on Tuesday
UAE Ramadan moon-sighting committee to meet on Tuesday
 RTA opens second bridge at Al Qudra intersection
RTA opens second bridge at Al Qudra intersection
 Abu Dhabi identifies over 40 modern heritage sites
Abu Dhabi identifies over 40 modern heritage sites
 Distribution of aid from UAE's Saqr Humanitarian Ship begins in Gaza
Distribution of aid from UAE's Saqr Humanitarian Ship begins in Gaza
